Surgical Mesh
Surgical mesh is used as additional support to weakened tissue. The mesh is made using synthetic or animal components. These meshes can be absorbable, non-absorbable, or a combination of both. Non – absorbable mesh stays in our body almost forever and is a permanent strengthening material of the tissue. BioCer’s TiO2 surgical mesh implant is a medical device used for hernia repair. Big market players of the surgical mesh implants are BioCer, Medtronic, Ethicon, and Bard.
Market Overview
The surgical meshes market is anticipated to gain market growth in the next 7 years. The market is forecasted to grow at a CAGR of 6.40% in the period. Growing awareness among the people concerning the gains of surgical meshes will create productive opportunities for the growth of the market.
With the expanding amount of applications in reconstructive operation such as pelvic organ prolapse, the surging volume of patients enduring from hernia and other conditions, handling of laparoscopic surgeries and minimally invasive procedures, the prevalence of improved healthcare infrastructure will heighten the growth of the surgical meshes market.
Factors affecting growth
The steep cost of operation accompanying by rigorous safety regulations and jeopardy of inflammatory reactions and infections are serving as market barriers for the growth of the surgical meshes.
Market Scope and Market Size
Based on product type
- non-absorbable surgical mesh,
- absorbable surgical mesh, and others.
Basis of application
- hernia repair,
- traumatic or surgical wounds,
- abdominal wall reconstruction,
- facial surgery.
Based on Selling medium
- direct channel
- distribution channel
Based on End-user
- hospitals
- ambulatory surgical centers,
- clinics, and others
Key Findings
- Inguinal hernia is the most common type of hernia complication. The number of people having to be treated by various treatment methods including surgical mesh is high. This complication treatment dominated the market 63%. This type of hernia is common among male and old people.
- Mesh material is a significant supporting segment in the hernia mesh market owing to the change in the treatment plan from autogenous tissue repair therapy to artificial and biological mesh to decrease the hazard of recurrence.
- Biological mesh is the most fast-growing segment in this market with healthcare providers moving towards this kind of treatment strategy
- The ambulatory surgical center is another growing end-user segment that is speculated to hold the market share for some percentage.
TiO2 Mesh and TiO2 Mesh Light

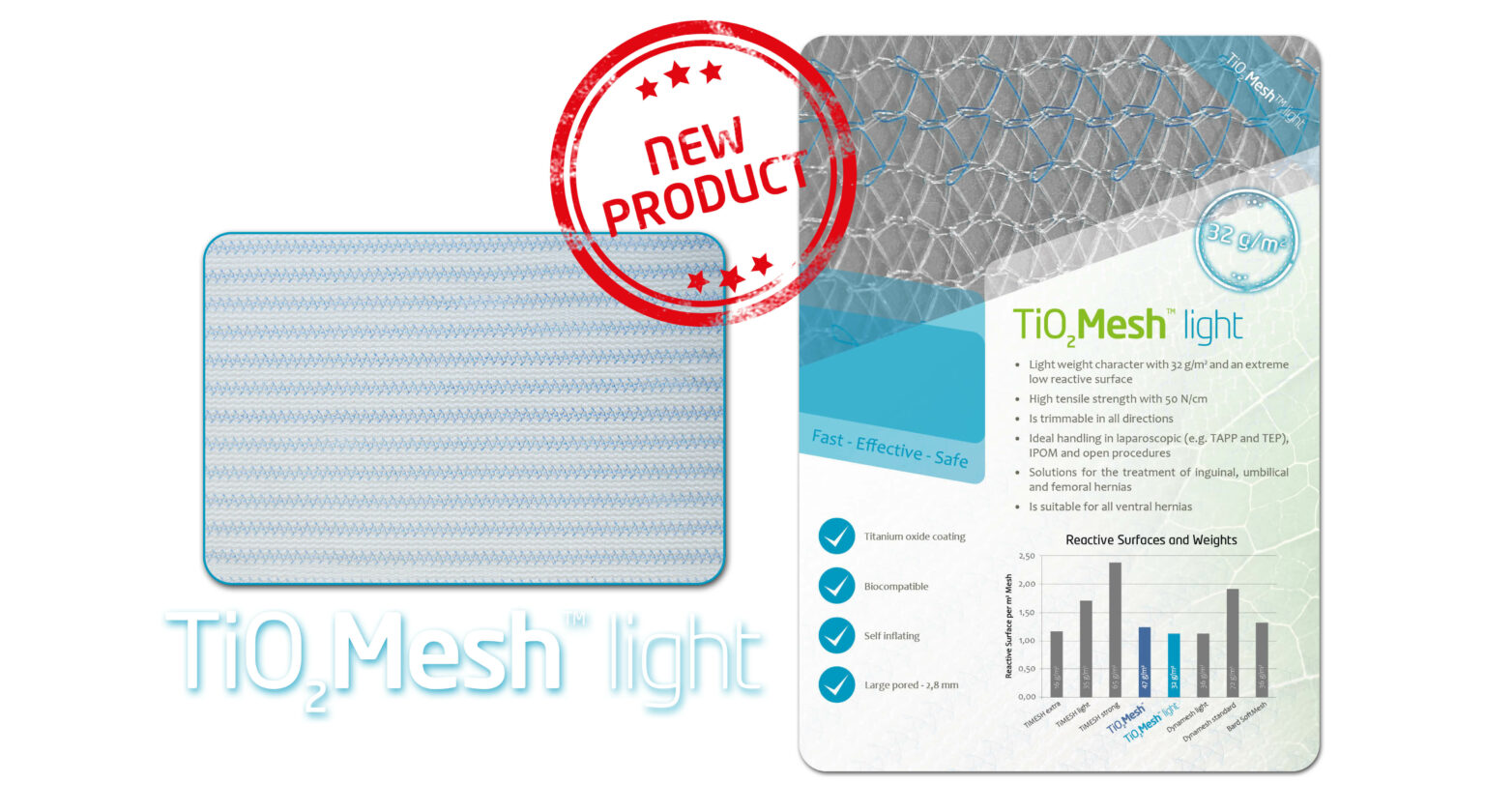
TiO2 surgical mesh implant as the name suggests is titanium oxide surgical hernia mesh specifically made to repair a hernia or soft tissue problem in the abdomen region. It is used for an added support. They are mainly used in the repair of inguinal and incisional hernias.
Morulaa Healthtech Pvt Ltd. is the licensed importer of BioCer products.
Areas of use
- This surgical mesh implant can be used for open and laparoscopic techniques mainly inguinal or incisional hernia repair.
- The high tensile strength of the mesh shows that it can handle various amounts of stress establishing its exemplary handling properties.
This information could be crucial for dealers and distributors as it will give them an idea of which kind of surgeons, hospitals to serve. From the areas of application here for TiO2 surgical mesh implants, dealers and distributors have great opportunities to market this surgical mesh implant.
Conclusion
TiO2 surgical mesh implant is a CE, CDSCO approved medical device. In this article, Global market of surgical mesh, the TiO2, surgical mesh information and areas of use of tche Morulaa licensed BioCer product is discussed. This Morulaa imported medical device is easy to use, safe and effective that can be used in different kinds of surgeries. It is a product imported by Morulaa and may be considered as a good alternative to other surgical mesh implants companies with products of, Medtronic, Ethicon, and Bard.
This article is intended to give product information on TiO2 surgical mesh implants in India. To know more about the product details, brochure and information about other medical devices please visit products.morulaa.com.
This information can be helpful to the dealers and distributors in India. Dealers and distributors reading this information can gain valuable understanding and details of the medical device.
Morulaa is a turnkey solution provider with an in-house Regulatory Consultancy team to conduct registrations of medical devices. Morulaa provides high-quality and professional services for Regulatory Consultancy and we aim at developing long-term relationships for the Indian Market.




